About project
Development of a new cardiosurgical device
HEART SENSE is an innovative probe that significantly increases safety during cardiac surgery. The main function of the HEART SENSE probe is to collect an electrical signal directly from the surface of the heart. This informs the team performing the operation about an increased risk of a critical event, especially a heart attack, in real time, allowing them to take pre-emptive actions and optimise the procedure. The HEART SENSE probe is for use primarily during coronary artery bypass surgery (ischaemic heart disease, coronary thrombosis, coronary atherosclerosis) without the use of extracorporeal circulation, i.e. on the beating heart.
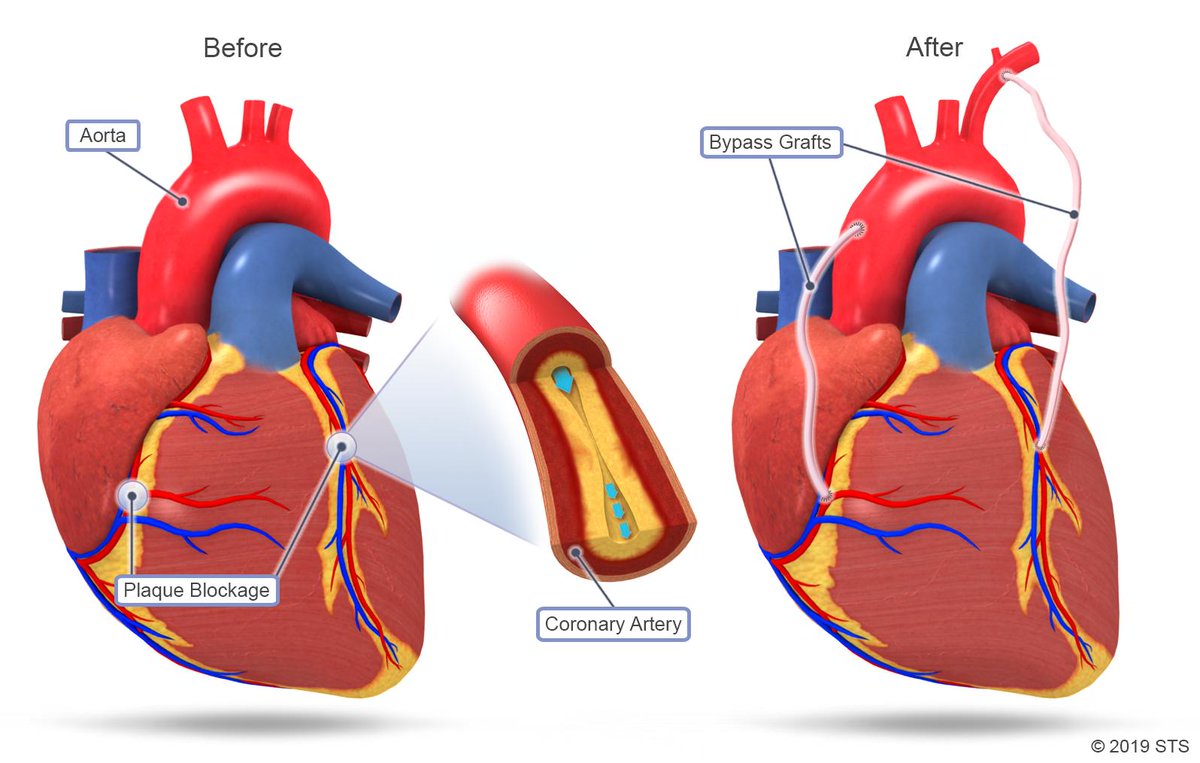
Coronary artery bypass surgeries
Modern medicine still cannot prevent the underlying process of atherosclerosis or cause regression of coronary artery disease. Coronary artery bypass grafting (CABG), i.e. the implantation of “bypasses”, is the most frequently performed cardiac surgery, with 833,063 operations, of this type, performed worldwide in 2016 and increasing to 876,420 in 2018 (source: GlobalData database). At the same time, publicly available results after many years of observation confirm that it is the most effective method for people with the most advanced stage of coronary thrombosis, in particular when compared to dilating the arteries with coronary stents.
Improving the results of beating-heart surgery
A growing number of reports call for improved CABG surgical techniques aimed at reducing the risk of complications, which result in both higher patient mortality and significant financial costs in Poland, ranging from tens to several hundred thousand zlotys per patient. Due to the global index of population growth and the increase in life expectancy, and a steady increase in the number of atherosclerosis cases, it can be assumed that this operation will be used for older and more vulnerable patients more likely to experience complications stemming from surgical treatment. Off-pump coronary artery bypass grafting (OPCAB) is less invasive compared to conventional coronary artery bypass surgery with extracorporeal circulation (i.e. the use of an artificial lung-heart to temporarily replace the functions of the heart and lungs) and less aggravating for elderly patients diagnosed with accompanying diseases such as decreased contractility, chronic kidney disease, lung diseases or stroke.
Strengthening the safety profile and improving the comfort of work for teams performing operations on the beating heart, through the use of the HEART SENSE medical device, may result in this surgical method gaining a clear advantage over techniques using the artificial lung-heart.
Extending the function of cardiac stabilisers
The HEART SENSE device can complement the functions of all cardiac stabilisers available on the market (Medtronic, Maquet, Terumo etc.). The HEART SENSE system does not require any modifications of the structure or use of cardiac stabilisers. When it is attached to the heart stabiliser (even in one package), HEART SENSE extends the functions of the stabiliser through the option to monitor heart rate.
Project stages
The originator of the device is cardiac surgeon Grzegorz Suwalski, MD. The concept has evolved into a technological and business project focused on optimal market implementation, through consultation with cardiac surgeons and anaesthesiologists, and also engineers, technologists, specialists in project management, technology transfer and certification, lawyers, patent agents and specialists in the med-tech and life science industries. After acceleration in the early development phase by BTM Innovations sp.z o.o. / Mazowiecki Klaster BioTechMed, in the first financial round investment in the project was made by the YouNick fund and Narodowe Centrum Badań i Rozwoju (the National Centre for Research and Development) as part of the Bridge Alfa program. The technology is fully owned by HEART SENSE. The first stage of the project will end as soon as the device enters the phase of clinical trials.
